Home
WA Newborn Bloodspot Screening Program
WA Newborn Bloodspot Screening Program
Newborn bloodspot screening (NBS) is a public health service that aims to protect babies from the effects of a range of treatable health conditions. It involves the collection of a blood sample shortly after birth that is tested to find out whether the baby has a condition for which early treatment can prevent intellectual or physical disability, or life-threatening complications. Around one in every thousand babies born in Australia will have one of the rare conditions tested for as part of newborn bloodspot screening.
Newborn bloodspot screening tests are provided free of charge. Screening is not compulsory, and parents may decline the test on behalf of their baby, although this could unnecessarily risk the baby's health. If you are a parent and would like to find out more about your baby’s newborn bloodspot screening test, visit the HealthyWA webpage.
About the WA NBS Program
Each year, the WA Newborn Bloodspot Screening Program tests approximately 35,000 newborns, representing over 99% of babies born in the state. Around 35 of these babies each year will be diagnosed with one of the conditions screened, enabling crucial treatment to be commenced before serious and often life-threatening problems occur.
To ensure babies with abnormal screening results receive timely diagnoses and treatment, the program collaborates with the Perth Children’s Hospital and Genetic Health WA as appropriate to the disorder.
The screening pathway
Before a baby can be screened, informed consent must be obtained from the baby’s parent or legal guardian. This involves a midwife or nurse providing information on the test to the family, who can then make a decision about whether the baby will be screened. Once the family has provided their consent, the baby's heel is pricked and a small sample of blood is collected. This usually occurs 48 to 72 hours after birth. The blood sample is then dried and mailed or couriered to the newborn bloodspot screening laboratory, where it is tested for a number of conditions.
If the screening test is abnormal, follow-up testing must be done to confirm a diagnosis. Most infants with abnormal screening results have normal follow-up testing. In these cases, initial screening results may have appeared abnormal because the blood was taken too early or the baby was premature, among many other factors. Once the diagnosis of a condition is confirmed, treatment starts immediately.
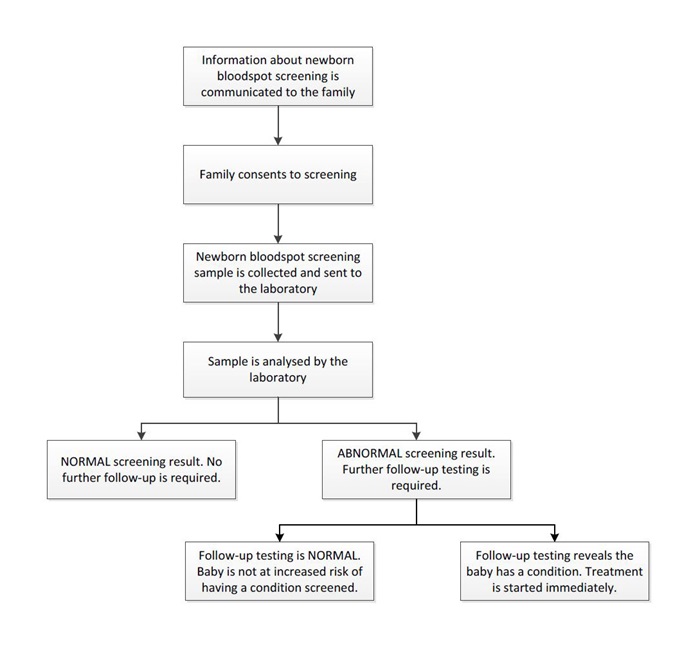
Figure 1. The Newborn bloodspot screening pathway
Screening limitations
Newborn bloodspot screening in Australia is of a very high quality. However, screening tests are not diagnostic. This means they only indicate whether an infant is at increased risk for a condition in the testing panel. In order to confirm whether an infant with an abnormal screening result actually has the condition, additional diagnostic testing is required.
The WA Newborn Bloodspot Screening Program has various quality assurance, educational and monitoring mechanisms in place to ensure that infants are appropriately screened and that the results are valid. While newborn bloodspot screening has proven reliable for the conditions it aims to detect, as with any laboratory test, false positive and false negative results are possible. For this reason, the possibility of a condition should never be ruled out solely based on the screening results and any signs or symptoms of a condition should be followed up immediately.
Newborn bloodspot screening in Australia
All Australian states and territories offer newborn bloodspot screening. The screening programs are guided by the Newborn Bloodspot Screening National Policy Framework 2018 (external site), which outlines the elements needed to successfully deliver newborn bloodspot screening in Australia. The Policy Framework provides advice in the following five areas:
- an overview of the programs, including their aim and objectives
- how the programs are implemented
- what is needed to support high-quality and safe newborn bloodspot screening
- how the programs should be monitored, reviewed and evaluated to ensure they are achieving their aim and objectives, and
- a decision-making process, to enable conditions to be assessed for inclusion in, or removal from, the programs.
The delivery of newborn bloodspot screening is also supported at a national level by the Human Genetics Society of Australasia’s policy on Recommendations for Screening for Specific Disorders (external site).
National program oversight
All State and Territory Health Ministers are involved in the national decision-making for newborn bloodspot screening in Australia. The decision-making process to add or remove conditions from programs currently uses the expertise of the Medical Services Advisory Committee, in line with the Newborn Bloodspot Screening National Policy Framework’s decision-making criteria (external site) . Please note that the Newborn Bloodspot Screening National Policy Framework and decision-making pathway is currently under review. For more information:
Local program oversight
The WA Newborn Bloodspot Screening Program activities are overseen by the WA Newborn Bloodspot Screening Committee. For more information, contact:
Office of Population Health Genomics
Public and Aboriginal Health Division, Western Australian Department of Health
Telephone: 08 9222 6888
E-mail: genomics@health.wa.gov.au
WA NBS Program annual outcome data
|
Program standard
|
2019
|
2020
|
2021
|
2022
|
|
Number of livebirths
|
33,145
|
32,027
|
34,481
|
32,301
|
|
Number of babies screened
|
99.6%
|
99.6%
|
99.6%
|
99.5%
|
|
Number of declines
|
45
|
56
|
51
|
102
|
|
Samples collected within 48-72 hours
|
88%
|
88%
|
85%
|
84%
|
|
Cards received by the laboratory within four days of an abnormal result
|
76%
|
65%
|
85%
|
82%
|
|
Babies referred for clinical assessment within three days of an abnormal result
|
94%
|
97%
|
94%
|
94%
|
|
Number of unsuitable samples
|
45
|
20
|
31
|
65
|
|
Total number of repeat samples requested
|
148
|
82
|
77
|
132
|
|
Total number of cases detected
|
40
|
37
|
40
|
53
|
Contact information
|
General enquiries
|
Phone (08) 6383 4171
Fax (08) 6383 4146
Email: wanbs@health.wa.gov.au
|
|
Senior Scientist
|
(08) 6383 4171
|
|
Clinical Director
|
(08) 6383 4103
|
|
Location
|
PathWest Laboratory Medicine WA
PP Block, QEII Medical Centre
Verdun St, Nedlands WA 6009
|
|
Postal address
|
PathWest Specimen Reception
PP Block, QEII Medical Centre
Locked Bag 2020, Nedlands WA 6909
|
Last reviewed: 07-01-2021